OTHER RESOURCES
KEY PAPER EVALUATION
Surface contamination monitoring in hospital pharmacy departmentsSurface contamination monitoring of hazardous drugs
The dangers of hazardous drug exposure
Exposure to hazardous drugs (HD) is a major concern for health care workers involved in HD handling, from preparation to administration. Points of hazardous drug contamination include HD receiving areas in the pharmacy, nursing or patient care units; floors under administration pumps; countertops, pass-throughs, storage trays and other equipment; locations where HD disposal bins are stored; and bathrooms used by patients or staff handling HDs.1
Occupational exposure of nurses and pharmacists to HDs can result in acute and chronic adverse events such as skin rashes, reproductive problems, and chromosomal aberrations.2 Although no cause-effect relationship has been definitively demonstrated between occupational exposure to HDs and the appearance of adverse events, it is generally believed that contamination levels should be kept as low as reasonably achievable (ALARA).2
The recognition of HD contamination and the associated health risks has led to the implementation of various measures to minimise HD exposure. Guidelines on the safe handling of HDs have been published by NIOSH, the American Society of Health-System Pharmacists, and the International Society of Oncology Pharmacy Practitioners.3-5 These guidelines recommend implementing vertical laminar airflow biological safety cabinets, providing personal protective equipment to workers, introducing closed-system transfer devices (CSTDs) for hazardous drug preparation and administration, and providing routine medical surveillance measures for staff. Despite clear demonstration that these methods reduce exposure to HDs, surface contamination is still a prevalent concern in hospital pharmacies and nursing units.6
The need for surface contamination monitoring
Monitoring surface contamination levels is essential for evaluating current HD handling practices and identifying opportunities for improvement. USP chapter 800 recommends that environmental wipe sampling for hazardous drug surface residue be performed at least every 6 months to evaluate the presence of contamination.7 However, USP <800> does not provide any guidance on how extensive the testing should be (e.g., how many locations or how many drugs should be tested). There is an overall lack of guidance on acceptable levels of contamination, but it has been established that routine monitoring of contamination sites is crucial in allowing institutions to be proactive in minimising hazardous drug contamination.6
One study investigated the surface contamination levels of 5 commonly used hazardous drugs (docetaxel, paclitaxel, cyclophosphamide, ifosfamide, and fluorouracil) in 338 hospital pharmacies.6 Wipe sampling was conducted at each pharmacy and sent to the lab for measurement of contamination levels.6 The study revealed that pharmacies with CSTDs had less surface contamination overall, but it was not completely eliminated (Figure 1).6
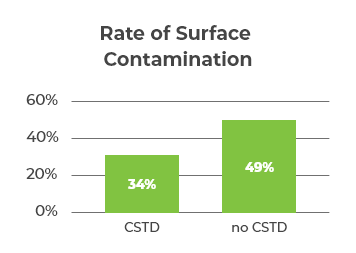
The study also showed a reduction in contamination levels between the first wipe event and subsequent wipe events, suggesting that contamination monitoring is beneficial in recognising and correcting practices.6 The lack of complete elimination of surface contamination indicated that monitoring needs to be implemented routinely and ongoingly in order to reach significantly low levels of surface contamination.6
Implementing a surface contamination programme
Despite the documented benefits of ongoing surface contamination monitoring, there are no standardised guidelines or procedures. The 2020 Safe to Touch consensus conference on HD surface contamination reported expert derived consensus statements on recommendations for managing surface contamination. The experts advised that institutions should implement a surface contamination monitoring plan and policy; employ routine testing and reporting of test results; implement safety practices; and conduct further research in HD surface contamination.1 A summary of their statements can be found here.
Surface contamination monitoring is an ongoing process. As such these recommendations require a committed multidisciplinary team for their successful implementation. Institutions should set short- and long-term goals for HD surface contamination monitoring. These goals should be specific, measurable, achievable, relevant, and time-bound, to implement best practices in their facilities.
References
1. Gabay M, Johnson P, Fanikos J, et al. Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. American Journal of Health-System Pharmacy 2021;78:1568-1575. Available at: https://academic.oup.com/ajhp/article/78/17/1568/6196020.
2. Valero-García S, González-Haba E, Gorgas-Torner MQ, et al. Monitoring contamination of hazardous drug compounding surfaces at hospital pharmacy departments. A consensus statement. Practice guidelines of the Spanish Society of Hospital Pharmacists (SEFH). Farmacia Hospitalaria 2021;45:96-107.
3. National Institute for Occupational Safety and Health. National Institute for Occupational Safety and Health Alert. Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. Cincinnati, OH; 2004.
4. Anon. ASHP Guidelines on Handling Hazardous Drugs. American Journal of Health-System Pharmacy 2006;63:1172-1191.
5. International Society of Oncology Pharmacy Practicioners Standards Committee. ISOPP standards of practice. Safe handling of cytotoxics. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners 2007;13 Suppl:1-81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17933809.
6. Salch SA, Zamboni WC, Zamboni BA, et al. Patterns and characteristics associated with surface contamination of hazardous drugs in hospital pharmacies. American Journal of Health-System Pharmacy 2019;76:591-598.
7. United States Pharmacopeial Convention. <800> Hazardous Drugs-Handling in Healthcare Settings. In: Rockville, Maryland: United States Pharmacopeia 42-National Formulary 37.; 2019.
8. Kiffmeyer TK, Tuerk J, Hahn M, et al. Application and Assessment of a Regular Environmental Monitoring of the Antineoplastic Drug Contamination Level in Pharmacies-The MEWIP Project. Ann Occup Hyg 2013;57:444-455. Available at: https://academic.oup.com/annweh/article/57/4/444/157902.