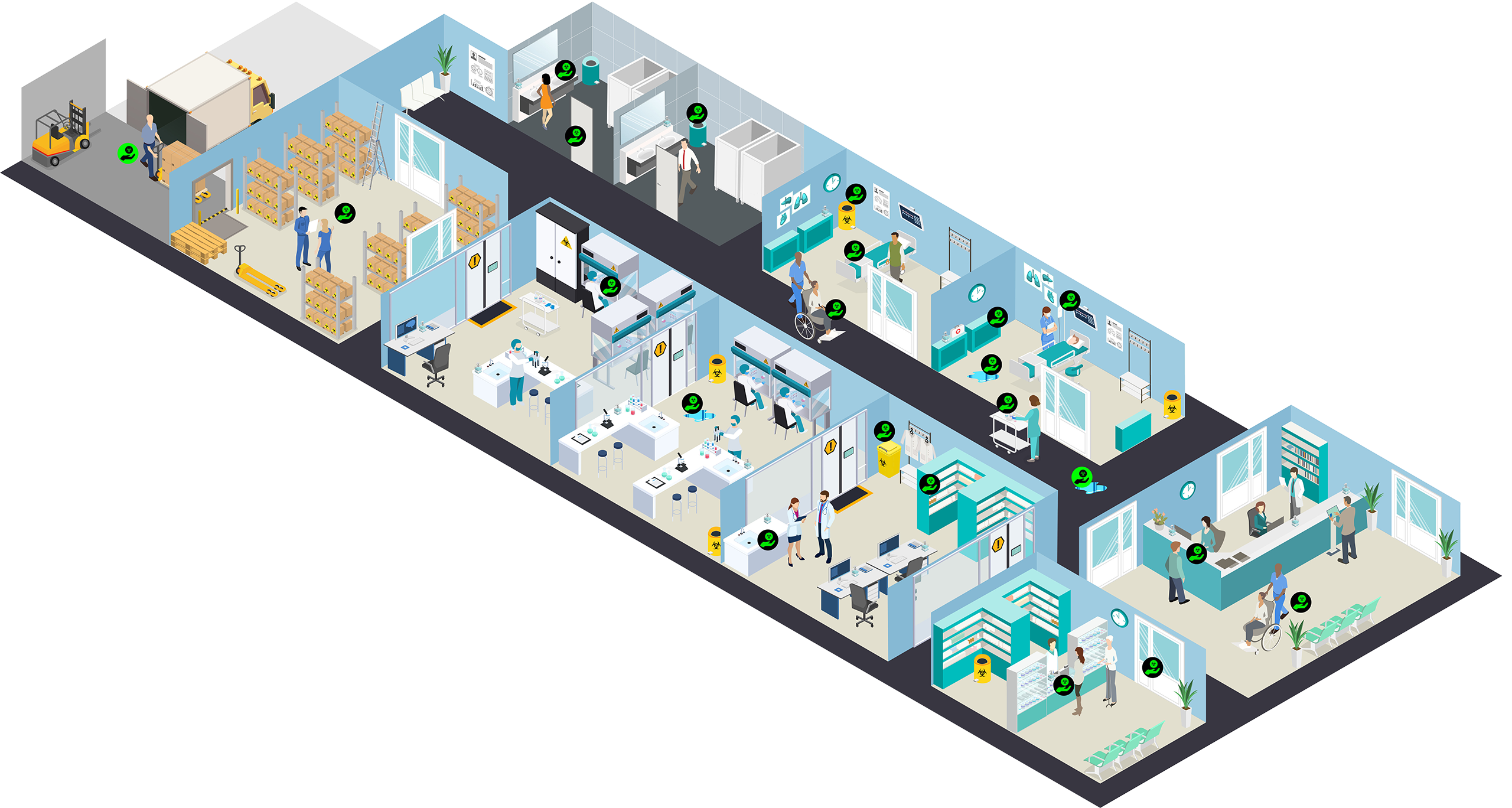
Contamination along the Care Continuum
Interactive Guide to the Guidelines
Loading dock
Hazardous drugs are transported from the loading dock into the storage area of the hospital pharmacy. Transportation of hazardous drugs causes the risk of exposure to all those in close proximity. Hazardous drugs include carcinogens, mutagens, reprotoxic substances, antiviral drugs, hormones, some bioengineered drugs, and other miscellaneous drugs (Article 11, DIRECTIVE 2004/37/EC, 2022) (NIOSH, 2016).
Guidelines
Hospitals should inform workers of containers containing carcinogens, mutagens or reprotoxic substances, ensure that all containers, packages, and installations containing carcinogens, mutagens or reprotoxic substances are labelled clearly and legibly, and display clearly visible warning and hazard signs (Article 11, DIRECTIVE 2004/37/EC, 2022).
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
NIOSH. (2016). NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016. https://www.cdc.gov/niosh/docs/2016-161/pdfs/2016-161.pdf
Resources
Reducing the Risk of Hazardous Drug Exposure to Health Care Workers.
https://safehandlingofhazardousdrugs.com/reducing-the-risk-of-hazardous-drug-exposure-to-health-care-workers
Storage of hazardous drugs (pre-preparation)
Hazardous drugs are stored on shelves in the pharmacy storage unit before they are prepared by the pharmacist. This can be the first point of exposure for pharmacists and other healthcare professionals as there could be leakage or traces of the drug on the packaging.
Guidelines
Hospitals should inform workers of containers containing carcinogens, mutagens or reprotoxic substances, ensure that all containers, packages, and installations containing carcinogens, mutagens or reprotoxic substances are labelled clearly and legibly, and display clearly visible warning and hazard signs (Article 11, DIRECTIVE 2004/37/EC, 2022).
Personal Protective Equipment (PPE) provides worker protection to reduce exposure to hazardous drug aerosols and residues. For hazardous drug handling outside of drug compounding, the hospital’s standard operating procedures (SOP) must describe the appropriate PPE to be worn based on its occupational safety plan and assessment of risk (Personal Protective Equipment, USP 800). The hospital must develop SOPs for PPE based on the risk of exposure and activities performed (Personal Protective Equipment, USP 800).
Disposable PPE must not be re-used. Reusable PPE must be decontaminated and cleaned after use (Personal Protective Equipment, USP 800).
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Reducing the Risk of Hazardous Drug Exposure to Health Care Workers.
https://safehandlingofhazardousdrugs.com/reducing-the-risk-of-hazardous-drug-exposure-to-health-care-workers/
Drug compounding
A hazardous drug is being prepared by compounding in a biological safety cabinet (BSC) by a pharmacist. During the compounding stage, the drug is out of packaging and exposed to personnel and the environment.
Guidelines
Engineering controls are required to protect the preparation from cross-contamination and microbial contamination (if preparation is intended to be sterile) during all phases of the compounding process (Compounding, USP 800).
A containment primary engineering control (C-PEC) is a ventilated device designed and operated to minimize worker and environmental exposures to hazardous drugs by controlling emissions of airborne contaminants (Compounding, USP 800).
A containment secondary engineering control (C-SEC) is the room with fixed walls in which the C-PEC is placed. It incorporates specific design and operational parameters required to contain the potential hazard within the compounding room (Compounding, USP 800).
Sterile and nonsterile hazardous drugs must be compounded within a C-PEC located in a C-SEC (Compounding, USP 800).
In, addition to USP 800, Nonsterile compounding and sterile compounding must follow standards in USP 795 and USP 797 respectively (Compounding, USP 800).
Requirements of CPEC and C-SEC for Nonsterile hazardous drug Compounding (Table 2, USP 800)
| C-PEC | C-SEC |
|---|---|
|
|
Requirements of CPEC and C-SEC for Sterile hazardous drug Compounding (Table 3, USP 800)
| C-PEC | C-SEC |
|---|---|
|
|
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
U.S. Pharmacopeia Convention (USP). (2019). USP General Chapter Pharmaceutical Compounding – Nonsterile Preparations. www.usp.org
U.S. Pharmacopeia Convention (USP). (2019). USP General Chapter Pharmaceutical Compounding – Sterile Preparations. www.usp.org
Resources
Reducing the Risk of Hazardous Drug Exposure to Health Care Workers.
https://safehandlingofhazardousdrugs.com/reducing-the-risk-of-hazardous-drug-exposure-to-health-care-workers/
Spills
A hazardous drug spill by a pharmacist has occurred during drug compounding. All surrounding healthcare workers are now at increased risk of exposure. During the transportation, preparation and administration of hazardous drugs, spills can occur resulting in further exposure of healthcare workers and patients.
Guidelines
Hospitals must develop a plan to prevent and clean hazardous drug spills. This plan should include who is responsible for spill clean-up, procedures for employees in-volved in the clean-up (e.g., PPE requirements, potential respirator usage) as well as those exposed, and information on spill kits (Safe to Touch Consensus, 2020).
- The 2018 “ASHP Guidelines on Handling Hazardous Drugs” contain appendices on recommendations for a spill clean-up procedure and contents of a hazardous drug spill kit (see resources)
In the event of a spill, the employer should inform the workers there of (Article 7, DIRECTIVE 2004/37/EC, 2022). Until the situation has been restored to normal and the causes of the abnormal exposure have been eliminated (Article 7, DIRECTIVE 2004/37/EC, 2022):
- only those workers who are essential to the carrying out of repairs and other necessary work shall be permitted to work in the affected area
- the workers concerned shall be provided with protective clothing and individual respiratory protection equipment which they must wear; the exposure may not be permanent and shall be kept to the strict minimum of time necessary for each worker
- unprotected workers shall not be allowed to work in the affected area
All personnel who may be required to clean up a spill of hazardous drugs must receive proper training in spill management and the use of PPE and NIOSH-certified respirators (Spill Control, USP 800).
References
Gabay, M., Johnson, P., Fanikos, J., Amerine, L., Kienle, P., Olsen, M., Roussel, C., & Moody, M. L. (2021). Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. American Journal of Health-System Pharmacy, 78(17), 1568–1575. https://doi.org/10.1093/ajhp/zxab134
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings. www.usp.org
Resources
Summary of 2020 Safe to Touch Consensus
https://safehandlingofhazardousdrugs.com/wp-content/uploads/2022/02/AP2176-SHHD-INFOGRAPHIC-v2.pdf
Surface Contamination Monitoring of Hazardous Dugs.
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Power, L. A., Coyne, J. W., & Hawkins, B. (2018). ASHP guidelines on handling hazardous drugs. In American Journal of Health-System Pharmacy (Vol. 75, Issue 24, pp. 1996–2031). American Society of Health-Systems Pharmacy.
https://doi.org/10.2146/ajhp180564
Sinks
A pharmacist has poured a hazardous drug down the sink. Incorrect disposal of hazardous drugs poses a threat of increased exposure to health care workers .
Guidelines
Hospitals must provide a means for safe collection, storage and disposal of waste by workers, including the use of sealed and clearly and visibly labelled containers. (Article 5, DIRECTIVE 2004/37/EC, 2022).
All personnel who perform routine custodial waste removal and cleaning activities in hazardous drug handling areas must be trained in appropriate procedures to protect themselves and the environment to prevent hazardous drug contamination. Disposal of all hazardous drug waste, including, but not limited to, unused HDs and trace-contaminated PPE and other materials, must comply with all applicable regional regulations (Disposal, USP 800).
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Reducing the risk of hazardous drug exposure to health care workers.
https://safehandlingofhazardousdrugs.com/reducing-the-risk-of-hazardous-drug-exposure-to-health-care-workers/
Prepared medication storage
Prepared medications are stored in shelves ready for distribution. Pharmacists and pharmacy technicians come into contact with these prepared hazardous drugs. Although they are in containers, they still pose a risk of exposure.
Guidelines
Hospitals should ensure that all containers, packages, and installations containing carcinogens, mutagens or reprotoxic substances are labelled clearly and legibly, and display clearly visible warning and hazard signs (Article 11, DIRECTIVE 2004/37/EC, 2022).
Hazardous drugs must be stored separately from non-hazardous drugs in a manner that prevents contamination and personnel exposure (Storage, USP 800, 2017).
Hazardous drugs must be stored in a manner that prevents spillage or breakage if the container falls. Do not store hazardous drugs on the floor. In areas prone to specific types of natural disasters (e.g., earthquakes) the manner of storage must meet applicable safety precautions, such as secure shelves with raised front lips. (Storage, USP 800, 2017).
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Bins
Bins for the disposal hazardous drug and equipment used for handling hazardous drugs are easily contaminated. If not used and disposed of appropriately, bins can present a significant hazardous drug exposure risk for maintenance personnel and other staff.
Guidelines
Hospitals should provide means for safe disposal of waste by workers, including the use of sealed and clearly visibly labelled waste containers (bins) (Article 5, DIRECTIVE 2004/37/EC,
2022).
Appropriate PPE such as eye and face masks must be worn when handling hazardous drugs during waste disposal (Eye and Face Protection, USP 800, 2017)
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Expert video on waste management of hazardous drugs:
https://safehandlingofhazardousdrugs.com/past-features/
Surfaces (counter top)
Traces of hazardous drugs can be inadvertently transferred to surfaces (e.g., countertops) by staff or patients who unknowingly have drug residue on their person. This puts other staff, patients, and visitors at risk.
Guidelines
Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every 6 months, or more often as needed, to verify containment) (Environmental and Quality Control, USP 800).
Surface wipe sampling should include (Environmental and Quality Control, USP 800):
- Interior of the C-PEC and equipment contained in it
- Pass-through chambers
- Surfaces in staging or work areas near the C-PEC
- Areas adjacent to C-PECs (e.g., floors directly under C-PEC, staging, and dispensing area)
- Areas immediately outside the hazardous drug buffer room
- Patient administration areas
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Surface Contamination Monitoring of Hazardous Drugs
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Surfaces (door handle)
Traces of hazardous drugs can be inadvertently transferred to surfaces (e.g., door handles) by staff or patients who unknowingly have drug residue on their person. This puts other staff, patients, and visitors at risk.
Guidelines
Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every 6 months, or more often as needed, to verify containment) (Environmental and Quality Control, USP 800).
Surface wipe sampling should include (Environmental and Quality Control, USP 800):
- Interior of the C-PEC and equipment contained in it
- Pass-through chambers
- Surfaces in staging or work areas near the C-PEC
- Areas adjacent to C-PECs (e.g., floors directly under C-PEC, staging, and dispensing area)
- Areas immediately outside the hazardous drug buffer room
- Patient administration areas
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings. www.usp.org
Resources
Surface Contamination Monitoring of Hazardous Drugs
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Patients and visitors
A patient is being pushed by staff to a patient room. Patients and visitors will likely not be as informed of the consequences of hazardous drug exposure so may be less inclined to adhere to precautions thus increasing their risk of adverse effects from hazardous drug exposure.
Guidelines
Areas for handling hazardous drugs must be located away from breakrooms and refreshment areas for personnel, patients, or visitors to reduce risk of exposure (Facilities and Engineering Controls, USP 800).
Designated areas must be available for (Facilities and Engineering Controls, USP 800):
- Receipt and unpacking
- Storage of hazardous drugs
- Nonsterile hazardous drug compounding
- Sterile hazardous drug compounding
All personnel who handle hazardous drugs are responsible for understanding the fundamental practices and precautions and for continually evaluating these procedures and the quality of final hazardous drugs to prevent harm to patients, minimise exposure to personnel, and minimise contamination of the work and patient-care environment (Responsibilities of Personnel Handling Hazardous Drugs, USP 800).
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs – Handling in Healthcare Settings.
www.usp.org
Patients and visitors
A hospital visitor is speaking to the receptionist. Should a hospital not adhere to safe hazardous drug handling standards, visitors will likely not be as informed of the consequences of hazardous drug exposure so may be less inclined to adhere to precautions thus increasing their risk of consequential adverse effects.
Guidelines
Areas for handling hazardous drugs must be located away from breakrooms and refreshment areas for personnel, patients, or visitors to reduce risk of exposure (Facilities and Engineering Controls, USP 800).
Designated areas must be available for (Facilities and Engineering Controls, USP 800):
- Receipt and unpacking
- Storage of hazardous drugs
- Nonsterile hazardous drug compounding
- Sterile hazardous drug compounding
All personnel who handle hazardous drugs are responsible for understanding the fundamental practices and precautions and for continually evaluating these procedures and the quality of final hazardous drugs to prevent harm to patients, minimise exposure to personnel, and minimise contamination of the work and patient-care environment (Responsibilities of Personnel Handling Hazardous Drugs, USP 800).
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Spills
A hazardous drug spill by a health care worker occurred during transportation to the wards. Surrounding staff and patients are now at increased risk of exposure. During the transportation, preparation and administration of hazardous drugs, spills can occur resulting in further exposure of healthcare workers and patients.
Guidelines
Hospitals must develop a plan to prevent and clean hazardous drug spills. This plan should include who is responsible for spill clean-up, procedures for employees in-volved in the clean-up (e.g., PPE requirements, potential respirator usage) as well as those exposed, and information on spill kits (Safe to Touch Consensus, 2020).
- The 2018 “ASHP Guidelines on Handling Hazardous Drugs” contain appendices on recommendations for a spill clean-up procedure and contents of a hazardous drug spill kit (see resources)
In the event of a spill, the employer should inform the workers there of (Article 7, DIRECTIVE 2004/37/EC, 2022). Until the situation has been restored to normal and the causes of the abnormal exposure have been eliminated (Article 7, DIRECTIVE 2004/37/EC, 2022):
- only those workers who are essential to the carrying out of repairs and other necessary work shall be permitted to work in the affected area
- the workers concerned shall be provided with protective clothing and individual respiratory protection equipment which they must wear; the exposure may not be permanent and shall be kept to the strict minimum of time necessary for each worker
- unprotected workers shall not be allowed to work in the affected area.
All personnel who may be required to clean up a spill of hazardous drugs must receive proper training in spill management and the use of PPE and NIOSH-certified respirators (Spill Control, USP 800).
References
Gabay, M., Johnson, P., Fanikos, J., Amerine, L., Kienle, P., Olsen, M., Roussel, C., & Moody, M. L. (2021). Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. American Journal of Health-System Pharmacy, 78(17), 1568–1575. https://doi.org/10.1093/ajhp/zxab134
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Summary of 2020 Safe to Touch Consensus
https://safehandlingofhazardousdrugs.com/wp-content/uploads/2022/02/AP2176-SHHD-INFOGRAPHIC-v2.pdf
Surface Contamination Monitoring of Hazardous Dugs.
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Power, L. A., Coyne, J. W., & Hawkins, B. (2018). ASHP guidelines on handling hazardous drugs. In American Journal of Health-System Pharmacy (Vol. 75, Issue 24, pp. 1996–2031). American Society of Health-Systems Pharmacy.
https://doi.org/10.2146/ajhp180564
Drug delivery to the wards
Hazardous drugs are transported to wards by a health care worker. Hazardous drug containers can fall and break as they are being transported around the hospital. As such more workers become exposed. If unaware that they are transporting hazardous drugs, health care workers may be less careful while transporting the hazardous drug.
Guidelines
Hospitals should inform workers of containers containing carcinogens, mutagens or reprotoxic substances, ensure that all containers, packages and installations containing carcinogens, mutagens or reprotoxic substances are labelled clearly and legibly, and display clearly visible warning and hazard signs (Article 11, DIRECTIVE 2004/37/EC, 2022).
Hazardous drugs must be transported in containers that minimise the risk of breakage or leakage. Pneumatic tubes must not be used to transport any liquid hazardous drugs or any antineoplastic hazardous drugs because of the potential for breakage and contamination (Transport, USP 800, 2017).
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Spills
A hazardous drug spill by a nurse has occurred during drug administration to a patient. Other patients and surrounding staff are now at increased risk of exposure to the drug. During the transportation, preparation and administration of hazardous drugs, spills can occur resulting in further exposure of healthcare workers and patients.
Guidelines
Hospitals must develop a plan to prevent and clean hazardous drug spills. This plan should include who is responsible for spill clean-up, procedures for employees in-volved in the clean-up (e.g., PPE requirements, potential respirator usage) as well as those exposed, and information on spill kits (Safe to Touch Consensus, 2020).
- The 2018 “ASHP Guidelines on Handling Hazardous Drugs” contain appendices on recommendations for a spill clean-up procedure and contents of a hazardous drug spill kit (see resources)
In the event of a spill, the employer should inform the workers there of (Article 7, DIRECTIVE 2004/37/EC, 2022). Until the situation has been restored to normal and the causes of the abnormal exposure have been eliminated (Article 7, DIRECTIVE 2004/37/EC, 2022):
- only those workers who are essential to the carrying out of repairs and other necessary work shall be permitted to work in the affected area
- the workers concerned shall be provided with protective clothing and individual respiratory protection equipment which they must wear; the exposure may not be permanent and shall be kept to the strict minimum of time necessary for each worker
- unprotected workers shall not be allowed to work in the affected area.
All personnel who may be required to clean up a spill of hazardous drugs must receive proper training in spill management and the use of PPE and NIOSH-certified respirators (Spill Control, USP 800).
References
Gabay, M., Johnson, P., Fanikos, J., Amerine, L., Kienle, P., Olsen, M., Roussel, C., & Moody, M. L. (2021). Report on 2020 Safe to Touch Consensus Conference on Hazardous Drug Surface Contamination. American Journal of Health-System Pharmacy, 78(17), 1568–1575.
https://doi.org/10.1093/ajhp/zxab134
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Summary of 2020 Safe to Touch Consensus
https://safehandlingofhazardousdrugs.com/wp-content/uploads/2022/02/AP2176-SHHD-INFOGRAPHIC-v2.pdf
Surface Contamination Monitoring of Hazardous Dugs.
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Power, L. A., Coyne, J. W., & Hawkins, B. (2018). ASHP guidelines on handling hazardous drugs. In American Journal of Health-System Pharmacy (Vol. 75, Issue 24, pp. 1996–2031). American Society of Health-Systems Pharmacy.
https://doi.org/10.2146/ajhp180564
Drug administration
A nurse is administering a carcinogenic drug to a patient. The drug is in close proximity to the nurse therefore increasing the risk of exposure. The drug could contact the nurse’s skin and result in adverse effects.
Guidelines
Appropriate PPE must be worn when administering hazardous drugs. Two pairs of chemotherapy gloves are required for administering antineoplastic, non-antineoplastics and re-productive risk only hazardous drugs (Personal Protective Equipment, USP 800,).
When chemotherapy gloves are required, they must meet American Society for Testing and Materials (ASTM) standard D6978 (see resources) (Personal Protective Equipment, USP 800, 2017).
Gloves must be powder-free because powder can contaminate the work area and can adsorb and retain hazardous drugs (Personal Protective Equipment, USP 800,).
Gloves must be inspected for physical defects before use. Do not use gloves with pin holes or weak spots (Personal Protective Equipment, USP 800,).
Hazardous drugs must be administered using protective medical devices such as needleless and closed systems and techniques such as spiking or priming of IV tubing with a non- hazardous drug solution in a C-PEC and crushing tablets in a plastic pouch (Administration, USP 800,).
After use, equipment and PPE must be removed and disposed of in a waste container approved for trace-contaminated hazardous drug waste at the site of drug administration (Administration, USP 800,).
Closed system transfer devices (CSTDs) must be used for administration of antineoplastic hazardous drugs when the dosage form allows (Administration, USP 800).
Administration into certain organs or body cavities (e.g., the bladder, eye, peritoneal cavity, or chest cavity) often requires equipment for which locking connections may not be readily available or possible (Administration, USP 800).
Healthcare personnel should avoid manipulating hazardous drugs such as crushing tablets or opening capsules if possible (Administration, USP 800). Liquid formulations are preferred if solid oral dosage forms are not appropriate for the patient (Administration, USP 800).
If hazardous drug dosage forms do require manipulation such as crushing tablet(s) or opening capsule(s) for a single dose, personnel must don appropriate PPE and use a plastic pouch to contain any dust or particles generated (Administration, USP 800).
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
ASTM standard D6978 https://www.astm.org/d6978-05r19.html
Surfaces (shelves and cupboards)
Traces of hazardous drugs can be inadvertently transferred to surfaces (e.g., shelves and cupboards) by staff or patients who unknowingly have drug residue on their person. This puts other staff, patients, and visitors at risk.
Guidelines
Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every 6 months, or more often as needed, to verify containment) (Environmental and Quality Control, USP 800).
Surface wipe sampling should include (Environmental and Quality Control, USP 800):
- Interior of the C-PEC and equipment contained in it
- Pass-through chambers
- Surfaces in staging or work areas near the C-PEC
- Areas adjacent to C-PECs (e.g., floors directly under C-PEC, staging, and dispensing area)
- Areas immediately outside the hazardous drug buffer room
- Patient administration areas
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs – Handling in Healthcare Settings.
www.usp.org
Resources
Surface Contamination Monitoring of Hazardous Drugs
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Patients and visitors
A patient is being pushed by staff to a patient room. Patients and visitors will likely not be as informed of the consequences of hazardous drug exposure so may be less inclined to adhere to precautions thus increasing their risk of adverse effects from hazardous drug exposure.
Guidelines
Areas for handling hazardous drugs must be located away from breakrooms and refreshment areas for personnel, patients, or visitors to reduce risk of exposure (Facilities and Engineering Controls, USP 800).
Designated areas must be available for (Facilities and Engineering Controls, USP 800):
- Receipt and unpacking
- Storage of hazardous drugs
- Nonsterile hazardous drug compounding
- Sterile hazardous drug compounding
All personnel who handle hazardous drugs are responsible for understanding the fundamental practices and precautions and for continually evaluating these procedures and the quality of final hazardous drugs to prevent harm to patients, minimise exposure to personnel, and minimise contamination of the work and patient-care environment (Responsibilities of Personnel Handling Hazardous Drugs, USP 800).
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs – Handling in Healthcare Settings.
www.usp.org
Surfaces (patient bed)
Traces of hazardous drugs can be inadvertently transferred to surfaces (e.g., patient beds) by staff or patients who unknowingly have drug residue on their person. This puts other staff, patients, and visitors at risk.
Guidelines
Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every 6 months, or more often as needed, to verify containment) (Environmental and Quality Control, USP 800).
Surface wipe sampling should include (Environmental and Quality Control, USP 800):
- Interior of the C-PEC and equipment contained in it
- Pass-through chambers
- Surfaces in staging or work areas near the C-PEC
- Areas adjacent to C-PECs (e.g., floors directly under C-PEC, staging, and dispensing area)
- Areas immediately outside the hazardous drug buffer room
- Patient administration areas
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Surface Contamination Monitoring of Hazardous Drugs
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Bins
Bins for the disposal hazardous drug and equipment used for handling hazardous drugs are easily contaminated. If not used and disposed of appropriately, bins can present a significant hazardous drug exposure risk for maintenance personnel and other staff.
Guidelines
Hospitals should provide means for safe disposal of waste by workers, including the use of sealed and clearly visibly labelled waste containers (bins) (Article 5, DIRECTIVE 2004/37/EC,
2022).
Appropriate PPE such as eye and face masks must be worn when handling hazardous drugs during waste disposal (Eye and Face Protection, USP 800, 2017)
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Expert video on waste management of hazardous drugs:
https://safehandlingofhazardousdrugs.com/past-features/
Bins
Hazardous drugs and equipment used for handling hazardous drugs should not be disposed of in just any bin. If not used and disposed of appropriately, bins can present a significant hazardous drug exposure risk for maintenance personnel and other staff.
Guidelines
Hospitals should provide means for safe disposal of waste by workers, including the use of sealed and clearly visibly labelled waste containers (bins) (Article 5, DIRECTIVE 2004/37/EC,
2022).
Appropriate PPE such as eye and face masks must be worn when handling hazardous drugs during waste disposal (Eye and Face Protection, USP 800, 2017)
References
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. (2022). DIRECTIVE 2004/37/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens, mutagens or reprotoxic substances at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC).
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Expert video on waste management of hazardous drugs:
https://safehandlingofhazardousdrugs.com/past-features/
Surfaces (door handle)
Traces of hazardous drugs can be inadvertently transferred to surfaces (e.g., door handles) by staff or patients who unknowingly have drug residue on their person. This puts other staff, patients, and visitors at risk
Guidelines
Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every 6 months, or more often as needed, to verify containment) (Environmental and Quality Control, USP 800).
Surface wipe sampling should include (Environmental and Quality Control, USP 800):
- Interior of the C-PEC and equipment contained in it
- Pass-through chambers
- Surfaces in staging or work areas near the C-PEC
- Areas adjacent to C-PECs (e.g., floors directly under C-PEC, staging, and dispensing area)
- Areas immediately outside the hazardous drug buffer room
- Patient administration areas
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs –Handling in Healthcare Settings.
www.usp.org
Resources
Surface Contamination Monitoring of Hazardous Drugs
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
Surfaces (restroom sink)
Traces of hazardous drugs can be inadvertently transferred to surfaces (e.g., bathroom sinks) by staff or patients who unknowingly have drug residue on their person. This puts other staff, patients, and visitors at risk.
Guidelines
Environmental wipe sampling for hazardous drug surface residue should be performed routinely (e.g., initially as a benchmark and at least every 6 months, or more often as needed, to verify containment) (Environmental and Quality Control, USP 800).
Surface wipe sampling should include (Environmental and Quality Control, USP 800):
- Interior of the C-PEC and equipment contained in it
- Pass-through chambers
- Surfaces in staging or work areas near the C-PEC
- Areas adjacent to C-PECs (e.g., floors directly under C-PEC, staging, and dispensing area)
- Areas immediately outside the hazardous drug buffer room
- Patient administration areas
References
U.S. Pharmacopeia Convention (USP). (2017). USP General Chapter Hazardous Drugs – Handling in Healthcare Settings.
www.usp.org
Resources
Surface Contamination Monitoring of Hazardous Drugs
https://safehandlingofhazardousdrugs.com/surface-contamination-monitoring-of-hazardous-drugs/
From delivery at the hospital to administration on the ward, there are many points at which healthcare workers, patients, and even their family members can be at risk of hazardous drug contamination.
This interactive tool reveals where contamination can occur, what the guidelines say about how to mitigate the risk of contamination, and provides direct links to more resources.
Click on one of the SHHD logos ![]() to learn more about contamination along the care continuum.
to learn more about contamination along the care continuum.
Best viewed on desktop