Guidelines & Directives
Evidence base for safe handling of hazardous drugs
Italian perspectives on the safe handling of hazardous drugs and closed systems
This infographic and key paper summary explains how safe handling of hazardous drugs is approached in Italy.
Restricted content
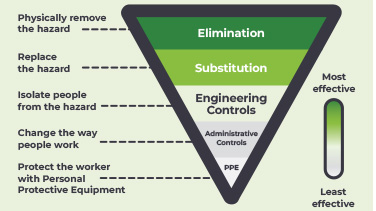
The Carcinogens, Mutagens and Reprotoxic Substances Directive (CMRD) is entering into law in April 2024. It contains guidance for employers on how to keep healthcare staff safe at work when handling hazardous medicinal products. Watch this conversation between Susanne Cruikshank and José Manuel Martínez Sesmero to understand more about the importance of the CMRD.
EU strengthens protection of healthcare workers from hazardous substances
This infographic explains the changes to the Carcinogens, Mutagens and Reprotoxic Substances Directive, and how this will affect healthcare workers.
Guidance for the management of hazardous medicinal products at work
This editorial summarises the appropriate topics for healthcare professionals from the European Commission’s recently published ‘Guidance for the safe management of hazardous medicinal products at work’.
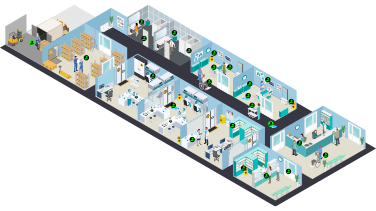
Contamination along the Care Continuum
This interactive pathway maps the key guidelines onto the various points of potential hazardous drug contamination in the hospital.
Carcinogens, Mutagens and Reprotoxic substances Directive (CMRD)
Updates to directive 2004/37/EC of the European Parliament and European Council were appoved on 8 March 2022. This 2-pager summarises the updates which are set to be released this year.
Hazardous Drug Exposure – summary of the guidelines
Hazardous drug handling is a complex, interdisciplinary process that requires adherance to guidelines and recommendations. This editorial dicsusses the scope of some of the various available guidelines and the importance of keeping up to date with their changes.
Restricted content
Compare how the “do’s and don’ts” of hazardous drug handling differ across Europe and discover how international directives could impact your working practice with this module. Watch Marta Trojniak introduce her updated module.