
Author: Marta Trojniak
Restricted content
Compare how the “do’s and don’ts” of hazardous drug handling differ across Europe and discover how international directives could impact your working practice with this module. Watch Marta Trojniak introduce her updated module.

Authors: Susanne Cruikshank & José Manuel Martínez Sesmero
Restricted content
The Carcinogens, Mutagens and Reprotoxic Substances Directive (CMRD) is entering into law in April 2024. It contains guidance for employers on how to keep healthcare staff safe at work when handling hazardous medicinal products. Watch this conversation between Susanne Cruikshank and José Manuel Martínez Sesmero to understand more about the importance of the CMRD.

Key Paper Evaluation
Carcinogens, Mutagens and Reprotoxic substances Directive (CMRD)
Updates to directive 2004/37/EC of the European Parliament and European Council were appoved on 8 March 2022. This 2-pager summarises the updates which are set to be released this year.
Editorial article
Clearing up the confusion – closed systems versus CSTDs
This short editorial summarises the definitions of closed systems versus closed system drug transfer devices.
Interactive guide to the guidelines
Contamination along the Care Continuum
This interactive pathway maps the key guidelines onto the various points of potential hazardous drug contamination in the hospital.
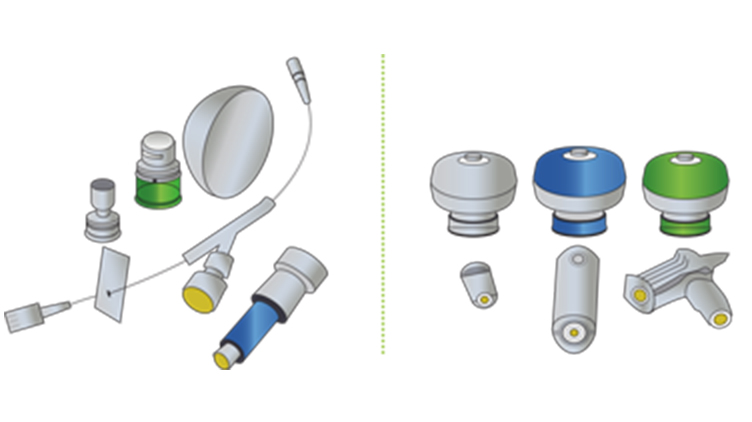
CSTDs vs Closed Systems
Engineering controls such as closed systems and CSTDs are essential for safe handling of hazardous drugs. This infographic illustrates the differences between closed systems and CSTDs.
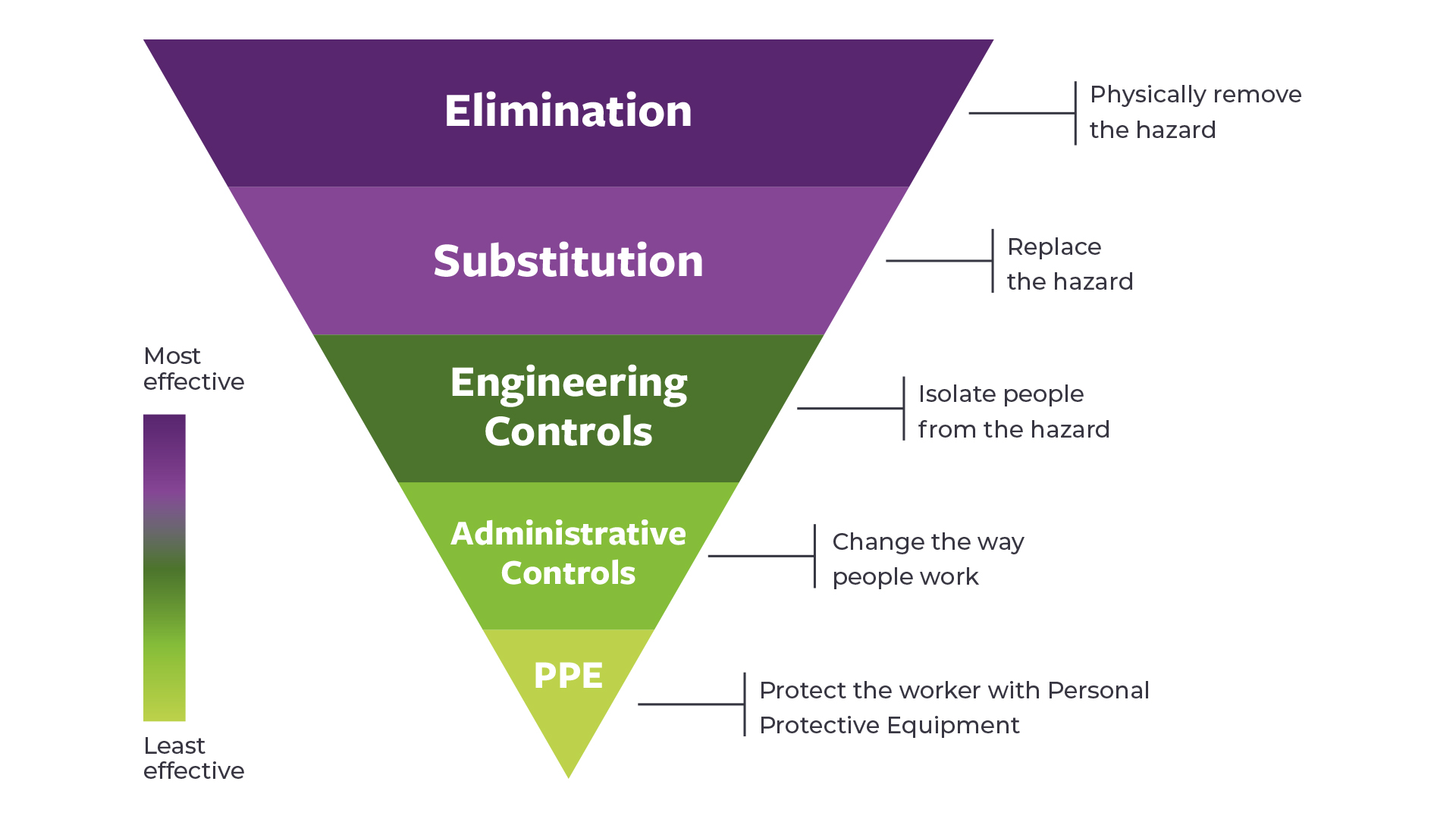
Key Paper Evaluation
Engineering controls for the safe administration of hazardous drugs
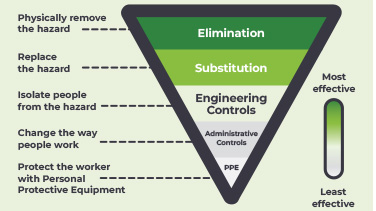
This one-pager summarises key information from three important documents for engineering controls: a joint position statement from the Oncology Nursing Society and the Hematology/Oncology Pharmacy Assocation, the USP <800>, and the NIOSH Hierarchy of Controls

Editorial article
Environmental Wipe Sampling – Personnel and Practices
This editorial explains who is responsible for monitoring surface contamination of hazardous drugs, and why it is so important
Infographic
EU strengthens protection of healthcare workers from hazardous substances

Authors: Alison Simons and Samantha Toland
Expert opinion: hazardous drug exposure of healthcare workers
The use of hazardous drugs (HD) for example cytotoxic drugs to treat cancer, has increased exponentially over the last 40 years. Sadly, however the awareness of the risks in administering HD has not increased at the same rate. Hear what two nurses have to say about the role of nurses in the handling of these drugs and what controls can be put in place to help mitigate the risks.