All Resources
Italian perspectives on the safe handling of hazardous drugs and closed systems
This infographic and key paper summary explains how safe handling of hazardous drugs is approached in Italy.
Restricted content
The Carcinogens, Mutagens and Reprotoxic Substances Directive (CMRD) is entering into law in April 2024. It contains guidance for employers on how to keep healthcare staff safe at work when handling hazardous medicinal products. Watch this conversation between Susanne Cruikshank and José Manuel Martínez Sesmero to understand more about the importance of the CMRD.
EU strengthens protection of healthcare workers from hazardous substances
This infographic explains the changes to the Carcinogens, Mutagens and Reprotoxic Substances Directive, and how this will affect healthcare workers.
Guidance for the management of hazardous medicinal products at work
This editorial summarises the appropriate topics for healthcare professionals from the European Commission’s recently published ‘Guidance for the safe management of hazardous medicinal products at work’.
Environmental Wipe Sampling – Personnel and Practices
This editorial explains who is responsible for monitoring surface contamination of hazardous drugs, and why it is so important
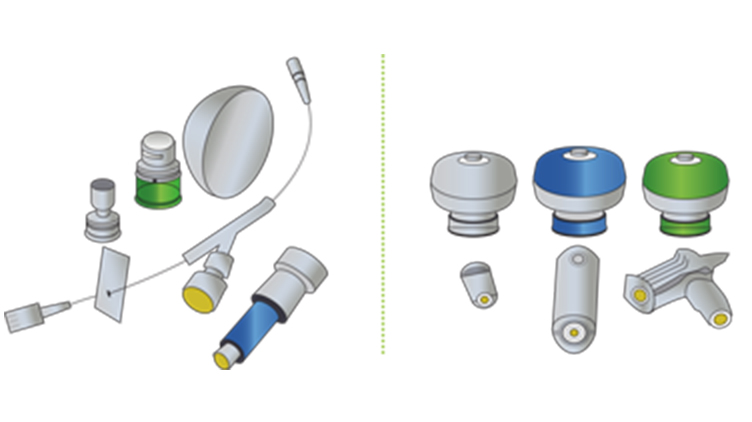
CSTDs vs Closed Systems
Engineering controls such as closed systems and CSTDs are essential for safe handling of hazardous drugs. This infographic illustrates the differences between closed systems and CSTDs.
Clearing up the confusion – closed systems versus CSTDs
This short editorial summarises the definitions of closed systems versus closed system drug transfer devices.
Precautions, perspectives, and practicalities: the reality of safe handling of hazardous drugs in hospitals
This short article summarises the main barriers to safe handling of hazardous drugs from the perspectives of nurses
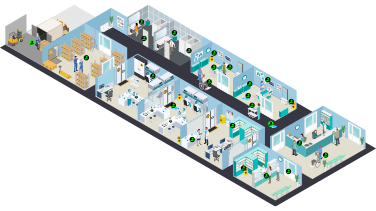
Contamination along the Care Continuum
This interactive pathway maps the key guidelines onto the various points of potential hazardous drug contamination in the hospital.
Carcinogens, Mutagens and Reprotoxic substances Directive (CMRD)
Updates to directive 2004/37/EC of the European Parliament and European Council were appoved on 8 March 2022. This 2-pager summarises the updates which are set to be released this year.
Hazardous Drug Exposure – summary of the guidelines
Hazardous drug handling is a complex, interdisciplinary process that requires adherance to guidelines and recommendations. This editorial dicsusses the scope of some of the various available guidelines and the importance of keeping up to date with their changes.
The Role of a Pharmacist in Patient Care – Medicine Management
In the first of 2 videos, Dr Sara Arenas discusses the role of the pharmacist in a multidisciplinary team. Pulling from her experience in medicine management, she describes the various aspects to consider in the financing and stocking of medicines.
The Role of a Pharmacist in Patient Care – Clinical Application
Dr Sara Arenas continues her discussion on the role of the pharmacist in a multidisciplinary team, specifically focusing on the vital clinical contribution pharmacists provide to patient care.
Waste Management of Hazardous Drugs
Watch Dr Christian Reiss discuss different waste management processes for hazardous drug disposal in three key areas of the drug pathway: the pharmacy, the ward and the patient’s home.
Monitoring contamination of hazardous drug compounding surfaces
This 2-page evaluation summarises key findings from a review of surface contamination monitoring in hospital pharmacy departments conducted by the Spanish Society of Hospital Pharmacists (SEFH).
Surface contamination monitoring of hazardous drugs
Surface contamination poses risks to healthcare workers and patients. In this month’s editorial, read about the importance of surface contamination monitoring to reduce hazardous drug exposure.
Reducing the risk of hazardous drug exposure to health care workers
One of the biggest concerns in the administration of hazardous drugs is the risk to those who come into contact with these drugs: patients, nurses and other health care professionals who administer the drug. Read this month’s editorial to explore ways of reducing these risks
Expert opinion: hazardous drug exposure of healthcare workers
The use of hazardous drugs (HD) for example cytotoxic drugs to treat cancer, has increased exponentially over the last 40 years. Sadly, however the awareness of the risks in administering HD has not increased at the same rate. Hear what two nurses have to say about the role of nurses in the handling of these drugs and what controls can be put in place to help mitigate the risks.
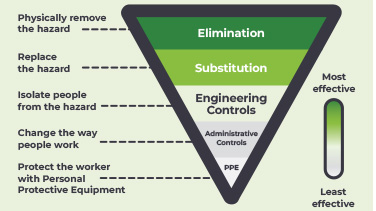
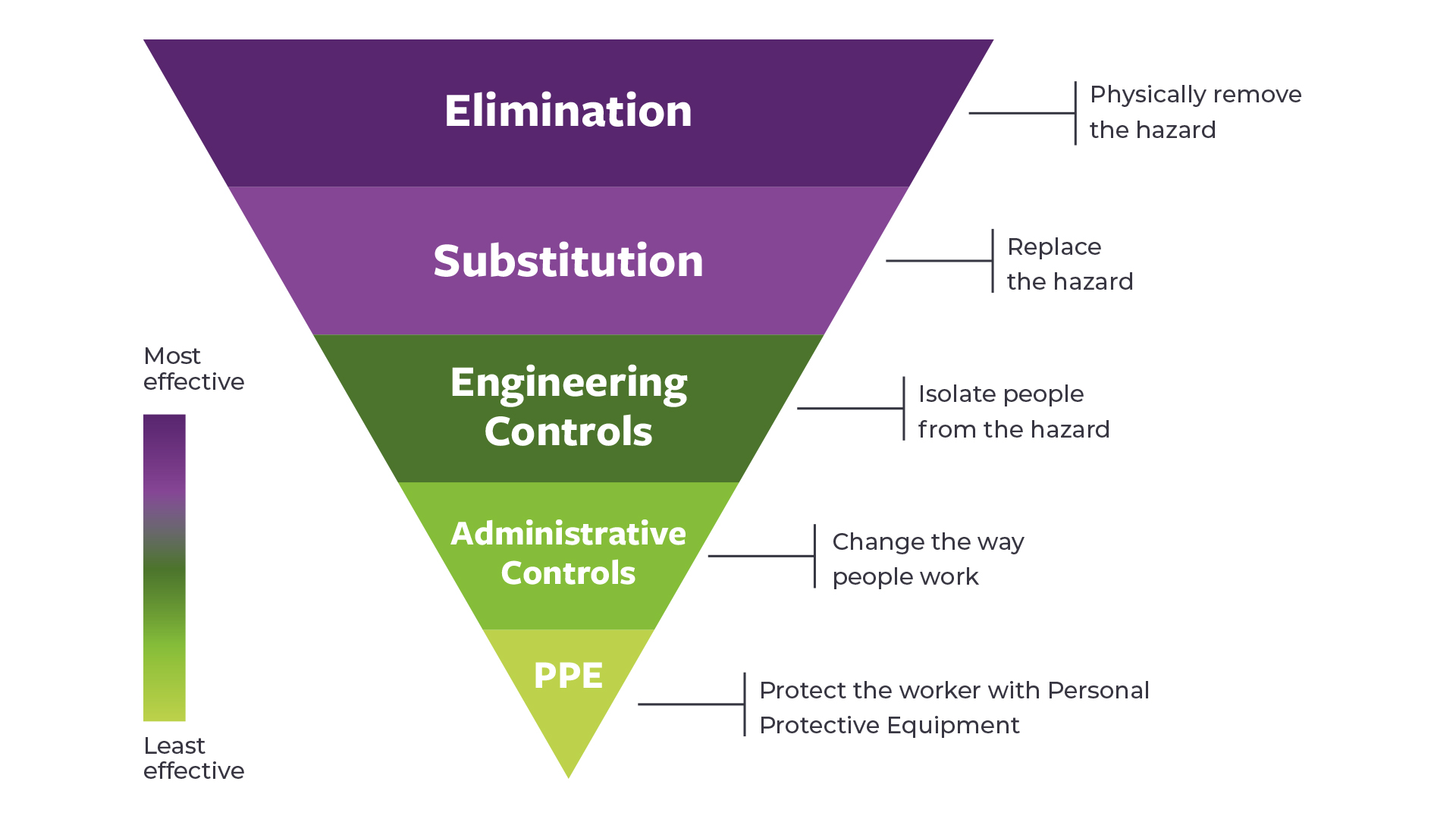
Engineering controls for the safe administration of hazardous drugs
This one-pager summarises key information from three important documents for engineering controls: a joint position statement from the Oncology Nursing Society and the Hematology/Oncology Pharmacy Assocation, the USP <800>, and the NIOSH Hierarchy of Controls
Repetitive Strain Injury Factsheet
Repetitive strain injury (RSI) is widespread in the medical workforce caused by repetitive movement or overuse. It can affect the muscles, nerves, or tendons mostly in the upper body. This factsheet provides an overview of recent facts and figures on the prevalence, risk factors, impact, and mitigation of RSI.
Restricted content
Dr Ana Herranz explores who is at risk of exposure to hazardous drugs, what precautions are put in place to protect them, who has responsibility for ensuring their safety and what guidelines and legislation exist to protect them. Watch her intro video or go direct to the module!
Restricted content
Compare how the “do’s and don’ts” of hazardous drug handling differ across Europe and discover how international directives could impact your working practice with this module. Watch Marta Trojniak introduce her updated module.
Restricted content
Dr Nicolas Simon introduces his updated module which delves into the problem of decontaminating antineoplastic drug compounding facilities.
Restricted content
Mark Santillo answers key questions on what constitutes acceptable hazardous drug risk assessment and highlights the most common failing. Watch the intro video or go direct to the module!
Restricted content
Hospital staff, patients and visitors are all affected if hazardous drugs are not handled safely. This module examines best practice in the preparation, administration and waste management of these drugs. Play the video to watch Johan Vandenbroucke introduce his updated module.
Restricted content
All healthcare professionals, and especially hospital pharmacists, need to concern themselves with ensuring the quality of injectable medications. This module explores the technologies used to maintain product integrity from preparation to administration. Watch Johan Vandenbrouke briefly introduce his updated module.
Restricted content
Some drugs are more hazardous than others, and appropriate levels of protection must be deployed by hospital employees responsible for ensuring safety. Watch Johan Vandenbroucke as he introduces this module about risk management strategies.